3720 Spectrum Blvd., Suite 125, Tampa, FL 33612
3720 Spectrum Blvd., Suite 125, Tampa, FL 33612
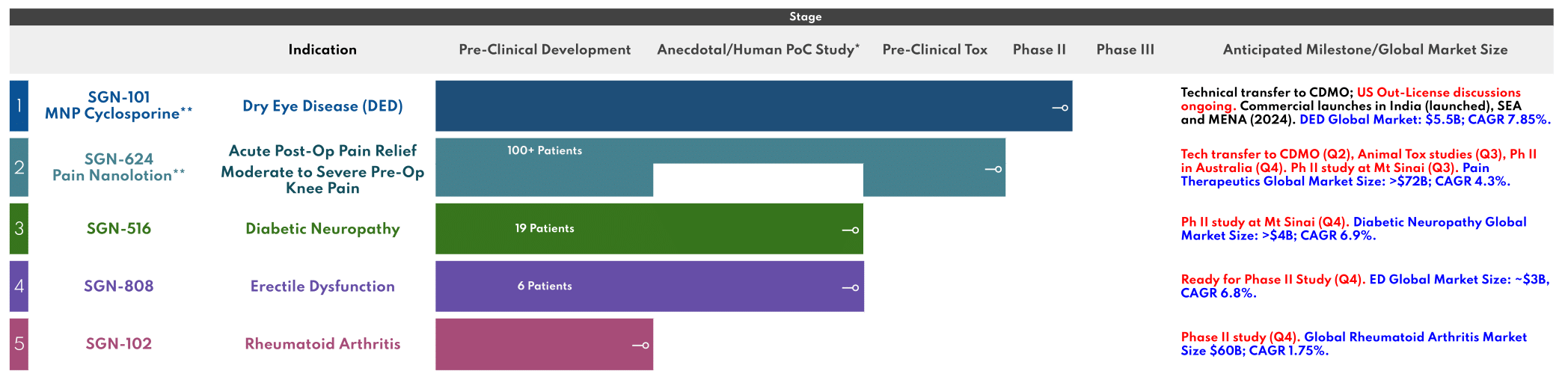
An overview of the products being researched, developed, and a brief summary of our pipeline.
Chronic diseases continue to be underserved by the current, prevalent mono-therapeutic approaches (which are partially effective at best), creating persisting unmet medical needs. Our objective is to select for and bring to market, clinically differentiated, superior combination nanomedicines that mitigate unmet medical needs in large therapeutic areas to create maximal patient impact.
* Human PoC – Human Proof-of-Concept Study, Open-label, Non-Randomized
** Pre-IND meeting with USFDA completed, feedback on Phase 3 Clinical Development plan obtained
In a study (published by BMC Ophthalmology) for Dry Eye Disease (DED), MNP Cyclosporine was (1) statistically superior to Restasis beginning 4 weeks of treatment and (2) more tolerable.
Current/Future Step(s):
Comprises of a transdermal, fixed-dose combination of two active pharma ingredients targeting inflammation and neuropathic components of pain. SGN-624 demonstrated significant efficacy in patients deemed “Medically Refractory” or whose pain was uncontrolled despite taking oral NSAIDs and Opioids in a 48-patient, single-center study. 16/25 patients in the study taking opioids either came off opioids completely or cut their dose in half after treatment for 1 month. Use of oral NSAIDs was also significantly and universally reduced. Overall, more than 50% of the patients experienced >50% improvement in pain reduction within two weeks of initiation of therapy.
Current/Future Step(s):
In a recently concluded 19-patient, two-center, open-label study of patients with uncontrolled diabetic neuropathy treated with SGN-516, 15/19 patients treated so saw a 50-80% improvement from baseline within one-month of treatment.
Current/Future Step(s):